Revolutionizing Hospital Disinfection
Nevoa is dedicated to revolutionizing the healthcare industry by providing technology-based solutions that automate disinfection and cleaning practices, ultimately reducing the risk of HAIs and saving lives.
Our Solutions
Features & Benefits
Features
- Automated disinfection technology
- Highly effective disinfection protocols
- Reduce overall cost compared to traditional manual cleaning methods
- Improve patient safety by reducing the risk of HAIs
- Innovative solution to a persistent problem in the healthcare industry
Benefits
- Significantly reduce the risk of hospital-acquired infections, including surgical site infections
- Improve patient outcomes and recovery rates
- Lower healthcare costs by reducing the need for extended hospital stays and additional treatments
- Enhance the overall quality of care provided by hospitals and healthcare facilities
- Increase the efficiency and productivity of hospital staff by automating disinfection protocols
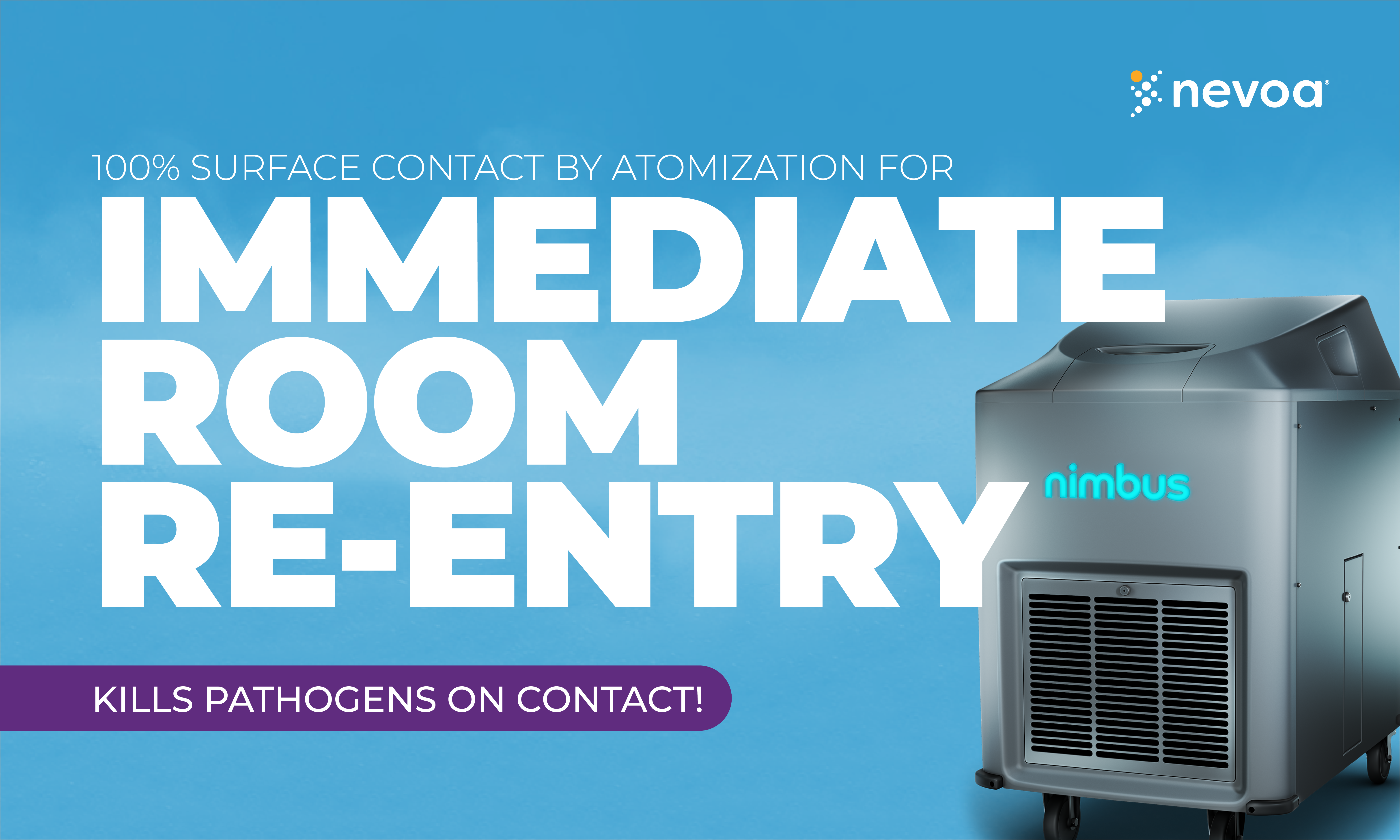
Nimbus
Our whole-room fogging technology is the only patented, hospital-grade surface disinfecting system approved for fogging with EPA-registered hypochlorous acid (HOCl).
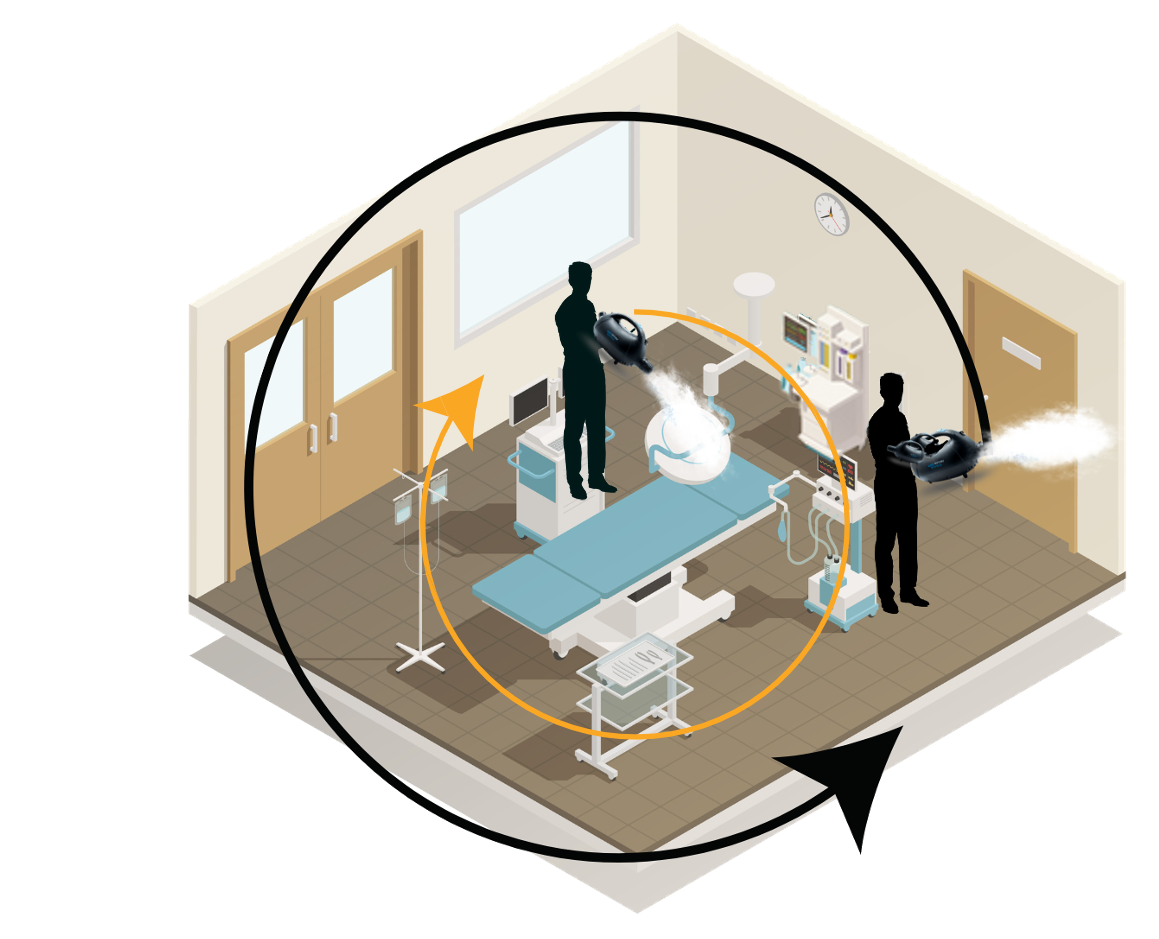
Stratus
Every patient deserves advanced disinfection. This lightweight handheld fogger makes between-case disinfection easy, efficient and highly effective in just two minutes.

Microburst
Nevoa’s proprietary hypochlorous acid solution is pH neutral, non-toxic and 80x – 100x more effective than bleach.

Why Partner with Nevoa?



